Protic Ionic Liquids
Recently, we have used OHD-OKE experiments and atomistic simulations to study protic ionic liquids, which are ionic liquids with an acidic proton on the cation. Protic ionic liquids have advantageous properties that make them ideal for applications in many fields including electrochemistry, biochemistry, and synthesis. They also have utility in carbon capture because hydrogen bonding has been shown to increase the solubility of CO2 in ionic liquids.

Figure 1.
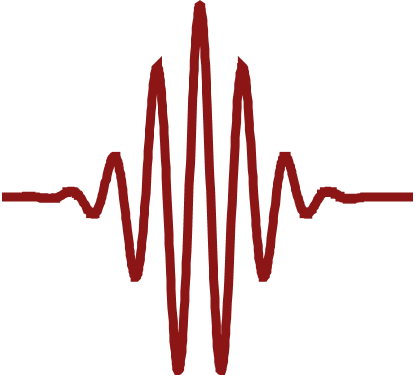
OHD-OKE experiments were conducted on two structurally analogous ionic liquids: an aprotic ionic liquid, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EmimNTf2, structure shown in Figure 1, R=CH3) and a protic ionic liquid, 1-ethylimidazolium bis(trifluoromethylsulfonyl)imide (EhimNTf2, structure shown in Figure 1, R=H). Ionic liquids are very hygroscopic and readily pick up water from the environment. Thus, in any application, there will be significant amounts of water in the ionic liquid. Therefore, it is important to study how water affects the structure and dynamics of protic ionic liquids. The experiments were conducted at multiple water concentrations ranging from dry to water saturated. The decays are shown in Figure 2a for EmimNTf2 and Figure 2b for EhimNTf2.

Figure 2.
The data presents as a series of power laws before a single exponential. Each functional form represents a unique randomization process, but the most interesting here is the final exponential. The time constant describing the exponential can be related to friction using
λi = kT/ηVDi
where λi is the friction coefficient about the ith axis, k is the Boltzmann constant, T is the temperature, V is the volume of the rotator, η is the viscosity, and Di is the orientational diffusion coefficient. The diffusion coefficient is equal to 1/6τ where τ is the final randomization time constant associated with the final exponential. The friction, normalized to slip boundary conditions, is plotted as a function of water concentration in Figure 3. The EmimNTf2 data is independent of water concentration which suggests that the ionic liquid is hydrodynamic and the addition of water does not fundamentally change the structure of the ionic liquid. EhimNTf2 (red) deviates more from slip boundary conditions than EmimNTf2 (black) indicating that Ehim+ must have stronger interactions with the surrounding molecules. The most interesting aspect of this plot is the deviation from linearity of the EhimNTf2 data. For samples up to 2:1 ion pair to water, the data is independent of water concentration, as seen in EmimNTf2. However, for samples with more water, there is a clear jump to higher friction. This indicates that the interactions between EhimNTf2 and neighboring species is fundamentally different at high water content. This was explored with atomistic simulations. The methodology for our atomistic simulations is described in the theory section of the website.

Figure 3.
Atomistic simulation results indicated that water molecules preferentially reside in the cavities between Ehim+ cations and NTf2- anions. This dispersed distribution of water among ionic species tends to break the directional hydrogen bonds between the Ehim+ cations and the NTf2- anions in order to form new hydrogen bonds between water molecules and neighboring ionic species. Water molecules serve as a bridge between the EhimNTf2 pairs previously in close contact. This complicates the local ionic environment as increasing amounts of water are added, resulting in new hydrogen bond networks forming among ionic groups.
The nature of the interactions fundamentally change between the 2:1 and 1:1 EhimNTf2:H2O samples. In a typical 2:1 sample, almost all water molecules are isolated between the Ehim+ cations and the neighboring NTf2- anions. Water-water hydrogen bonds are rare. The bonding of water's oxygen to the nitrogen-bound hydrogen atom of Ehim+ is comparable in strength to the bonding of a water hydroxyl to a water oxygen due to the lack of water-water hydrogen bonds at this low water concentration.
Increasing the water concentration from 2:1 to 1:1 EhimNTf2:water leads to a dramatic change in water-water hydrogen bonding interactions. A further introduction of water molecules into this sample leads to a distinct aggregation of water molecules in ionic cavities. This was experimentally confirmed with FT-IR spectra of the deuterated ionic liquid. These water pairs and clusters, which will be even more prevalent at the 0:65:1 concentration, will further reduce the Coulombic interactions among cations and anions, and replace them with hydrogen bonds. This change in the structural environment of the cation contributes to the change in the dynamical behavior of the ionic liquid as measured by OHD-OKE.
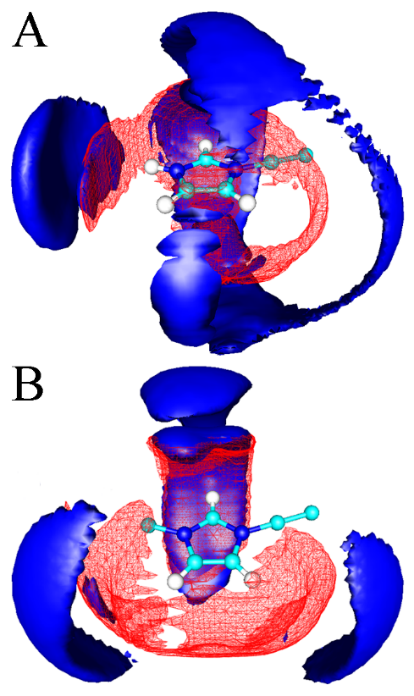
Figure 4.
Atomistic simulations were also used to explore protic and aprotic ionic liquids in the absence of water. Hydrogen bonding is a key driving force for the difference of liquid viscosities for neat EhimNTf2 and EmimNTf2 ionic liquids. The viscosities are 57.1 cP and 36.3 cP, respectively. Atomistic simulation results indicated that the H5 atoms (as labeled in Figure 1) in both the Ehim+ and Emim+ cations, and the N-H sites in the Ehim+ cation, exhibit prominent hydrogen bonding interactions with the N and O atoms in the NTf2- anions, whereas the H4 atoms in the two cations only present considerable hydrogen bonding interactions with the O atoms in the NTf2- anions. Such a coordination feature is manifested in spatial distribution functions of N (solid blue surface) and O (meshed red surface) atoms in NTf2- anions around a central Ehim+ (Figure 4A) and Emim+ cation (Figure 4B). The O atoms in the NTf2- anions prefer to form multiple hydrogen bonds with all the hydrogen atoms on the imidazolium rings leading to their diffusive feature around the imidazolium ring in the Ehim+ cation. The N atoms in the NTf2- anions are mainly localized around the N-H and H5 sites resulting in their discrete distributions near H4 atoms. However, the O atoms in the NTf2- anions are localized in the first solvation shell of the neighboring H4 and H5 atoms on the imidazolium rings of the Emim+ cation. The N atoms in the NTf2- anions exhibit two distinct distribution domains along the CR-H5 vector, contributing to direct hydrogen bonding interactions between H5 and N atoms. The increasing complexity of the hydrogen bonding network in EhimNTf2 results in a higher viscosity.
Relevant Publications
468. "The Impact of Hydrogen Bonding on the Dynamics and Structure of Protic Ionic Liquid/Water Binary Mixtures," Heather E. Bailey, Yong-Lei Wang and Michael D. Fayer J. Phys. Chem. B DOI: 10.1021/acs.jpcb.7b06376 (2017).